Microfluidic device intergrading a selectively permeable membrane and a 3D vascular network for the in vitro development of anatomical-functional barrier
3D microfluidic platform with a biomimetic blood vessel network (CVN) mimicking the choroidal vascular network covered by a bioengineered Bruch’s membrane (BrM).
BACKGROUND
Age-related macular degeneration (AMD) is a multifactorial chronic eye disease and the leading cause of blindness in millions of elderly people world-wide. In AMD patients, several morphological and structural changes occur in the retinal pigment epithelium (RPE), the Bruch’s membrane (BrM), and the choriocapillaris resulting in the progressive vision loss. At present, the exact disease pathogenesis remains poorly understood. As a result, no effective treatments exist to halt or reverse their progression. To investigate the pathophysiological process underlying AMD and to validate novel drug candidates, several in vitro models have been proposed, but they are limited to a single cell type and they lack physiological realism and great predictive value.
This device mimics the ocular interface composed of Choroid Vascular Network (CVN) and RPE, thus meeting the need for a multifaceted BrM technological system which can be applied in the pharmaceutical industry.
TECHNOLOGY OVERVIEW
A prototype of the present invention of the type “organ-on-a-chip” was validated in the laboratory for the in vitro development of the blood-retinal barrier (Figure 1). Namely, to mimic the CVN, a microfluidic network was designed starting from Optical Coherence Tomography scans performed in human retina, and fabricated on polydimethylsiloxane (PDMS) through a novel manufacturing method established to provide a time-saving and cost-effective alternative to the common lithographic-based techniques. The interior surfaces of the microfluidic channels were subsequently coated with chemically crosslinked gelatin to promote cell adhesion and long-term culture. Perfusion tests were successfully performed to validate the overall microfluidic platform. This microfluidic devise was then tested: Human Umbilical Vein Endothelial cells (HUVECs) were cultured on the engineered PDMS-gelatin substrates to evaluate cells adhesion and proliferation under static conditions. HUVECs were shown to adhere and proliferate on the PDMS-gelatin substrates.
In parallel, electrospun gelatine mats and GelMA-coated electrospun mats were produced on top of PDMS films and tested for hESc-RPE cells culture. These cells were able to adhere and form complete cobblestone monolayers.
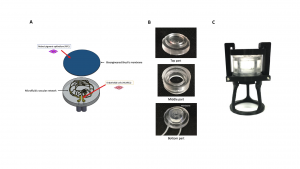
Figure 1: Bioengineered 3D in vitro model of the posterior ocular barrier. A) Schematic of the in vitro model, in which all the functional parts are illustrated. Once the bioengineered Bruch’s membrane and the microfluidic vascular network (MVN) are assembled, the complete structure will permit the co-culture of endothelial cells and RPE cells in a dynamic environment more similar to the natural one. B) Demolded parts of the final bioreactor prototype. C) Bioreactor prototype assembled with the locking system and the support structure.
STAGE OF DEVELOPMENT
Technology Readiness Level (TRL): 3 – Experimental Proof of Concept
BENEFITS
The retina is a complex structure difficult to reproduce in vitro. Researchers and pharma companies are then constrained to simplified in vitro models or to the use of animal models. Our device mimics the ocular interface composed of Choroid Vascular Network (CVN) and RPE, thus meeting the need for a multifaceted BrM technological system.
APPLICATIONS
This model of the blood-retinal barrier can be used on drug screening for AMD or other retinal diseases, by companies operating in the biomedical, pharmacological and biotechnology sectors.
INTELLECTUAL PROPERTY
- IT102021000032849 (Priority Date: 29/11/2021).
OPPORTUNITY
The present invention is thought to have the following economic potential:
- Establishment of an innovative start-up in biotechnology and bioengineering;
- Obtaining proceeds from the sale of the patent to companies operating in the biomedical, pharmacological and biotechnology sectors;
- Attracting investors in the biotechnology field of bioengineering;
- Obtaining proceeds from the granting of the license (sole, exclusive or non-exclusive) of the patent for purposes agreed with third parties.
NOVA Inventors
Sandra Tenreiro
Miguel Seabra


