Novel and innovative D-allose synthesis method
Chemo-enzymatic approach for the synthesis of rare C3-sugars using glycoside 3-oxidase (engineered) enzymes
BACKGROUND
Rare sugars, a diverse group of naturally occurring carbohydrates found in small quantities, are attracting increasing attention for their unique functional properties and potential health benefits. They are versatile compounds with applications as food additives, nutraceuticals, and active pharmaceutical ingredients. This category includes hexoses like D-allose, D-allulose, and D-tagatose; pentoses such as D-lyxose and L-xylulose; and disaccharides like turanose and isomaltulose.
One of the primary challenges in harnessing the potential of rare sugars is their low natural abundance, which restricts large-scale extraction and application. This scarcity has spurred the development of synthetic production methods, but existing approaches often fall short. Chemical synthesis, while capable of achieving viable yields, is expensive and environmentally harmful due to the use of costly catalysts and the generation of hazardous waste.
Enzymatic methods, particularly those based on the Izumoring strategy, offer a more eco-friendly alternative. However, these methods typically yield low amounts of the target sugars and require extensive purification, limiting their practicality for industrial-scale production.
To overcome these challenges, we have developed a novel chemo-enzymatic approach that is both efficient and scalable. The process includes an initial selective enzymatic oxidation step that produces no byproducts, followed by two successive chemical reactions using readily available reagents. The absence of byproducts in the overall process significantly reduces the need for extensive purification, representing a substantial improvement over current methods. Additionally, the affordability of the starting materials, the irreversibility of the enzymatic oxidation, and the high yields achieved in the stereoselective reduction make this approach a sustainable, scalable and cost-effective solution.
TECHNOLOGY OVERVIEW
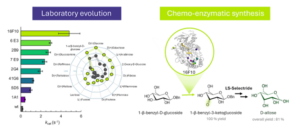
Using a sustainable, eco-friendly method, we propose a four-step chemoenzymatic process for synthesizing D-allose from minimally protected benzyl-glucose. This innovative strategy is the first reported approach requiring one enzymatic step: regio-selective oxidation at C3 of benzyl-glucosid performed by an engineered bacterial glycoside-3-oxidase using directed evolution approaches. This enzyme, named 16F10, shows improved 10-fold kinetic stability, 20-fold higher activity for D-glucose and 5-fold improved catalytic efficiency for 1-O-benzyl-α/β-D-glucopyranoside and 1-O-benzyl-β-D-glucopyranoside when compared to the wild-type enzyme. The oxidation of the minimally protected 1-O-benzyl-β-D-glucopyranoside by the enzyme occurs regioselectively at C3 with 100 % yields. Although the enzyme utilizes the 𝛼-anomer, it shows a higher specificity for the 𝛽-anomer. The second step of the reaction is the chemical reduction of the ketone at C-3 of the oxidised product using a commercial reagent (LS-selectride) to afford the alcohol with inverted configuration at C-3 in a well-known process, affording the allose derivative in 86% yield. Finally, hydrogenation cleaves the benzyl protecting group to afford the rare sugar D-allose in 94% yield. This approach also holds promise for the synthesis of other rare C3 epimers of sugars.
STAGE OF DEVELOPMENT
TRL 3 – experimental proof of concept
BENEFITS & APPLICATIONS
The novel synthetic route for D-allose and other rare sugars developed in this study has significant commercial applications, particularly in the food, pharmaceutical, chemical and nutraceutical industries. The ability to produce D-allose efficiently and at lower costs can lead to its incorporation into a wide range of products.
In the food industry, D-allose can be marketed as a non-caloric sweetener in various food and beverage formulations, catering to health-conscious consumers and those with dietary restrictions, such as diabetics. Products could include low-sugar snacks, beverages, and desserts, highlighting D-allose’s unique sweetening properties without contributing to caloric intake.
D-allose has been gaining significant attention for its beneficial properties as anticancer, antioxidant, anti-inflammatory, anti-hypertensive and immunosuppressor agent. D-allose can be formulated into dietary supplements or functional foods due to its potential health benefits, in the pharmaceutical and nutraceutical sectors. These products could target individuals seeking to enhance their overall health, manage specific medical conditions, or improve metabolic health.
Despite its various benefits, D-allose remains a minor player in the industrial market due to its limited availability and high production costs. Its efficient chemoenzymatic synthesis process sets this invention apart from existing products and methods; studies exploring the enzymatic synthesis of C3 compared to C2 epimeric rare sugars are more scarce. Traditional methods often involve lengthy and costly procedures that yield low amounts of product and undesirable by-products. In contrast, our method utilizes an engineered enzyme that significantly improves catalytic efficiency and operational stability, resulting in higher yields and purer products with fewer purification steps. This innovative approach enhances product availability and creates a competitive edge in the rapidly growing market for healthier food and dietary products.
INTELLECTUAL PROPERTY
- Provisional Patent Application Submitted (PT119793)
OPPORTUNITY
Licensing
Commercial Partner
Our aim is to license our technology to partners for commercialization in industries specialized in rare sugar production, with applications in food and pharmaceuticals.
NOVA Inventors
Lígia Martins
André Taborda
Rita Ventura
Márcia Rénio


