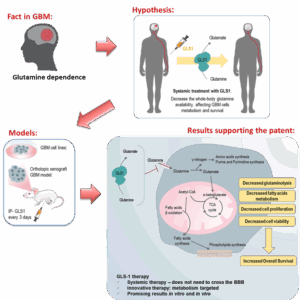
Glutaminase systemic application: a new tool to treat glioblastoma (GBM)
Systemic glutaminase therapy targeting GBM metabolism, reducing tumor viability avoiding the BBB hurdle.
BACKGROUND
Glioblastoma (GBM) is the most aggressive and treatment-resistant brain tumor, accounting for 54% of gliomas and 16% of all primary brain tumors. With a median survival of just 8 months and a 93% five-year mortality rate, current treatment options—including surgery, radiotherapy, and chemotherapy—offer limited effectiveness. GBM’s adaptability, redundancy in glutamine transport mechanisms, and reliance on external glutamine uptake make it highly resistant to traditional therapies.
Despite significant R&D investments, most experimental therapies fail due to blood-brain barrier (BBB) penetration issues and tumor metabolic plasticity. The high economic burden, with treatment costs ranging from $95,000 to $268,000 per patient annually, underscores the need for innovative, effective, and scalable therapeutic solutions.
Our novel systemic glutaminase (GLS1) therapy offers a paradigm shift in GBM treatment, targeting tumor metabolic dependence on glutamine rather than relying on direct tumor penetration. By acting in the bloodstream to deplete circulating glutamine, this approach starves tumor cells while sparing healthy tissues, presenting a disruptive and more effective alternative to current GBM therapies.
TECHNOLOGY OVERVIEW
Our biologics-based systemic therapy exploits GBM’s unique glutamine dependency, a critical metabolic vulnerability. Unlike conventional treatments that require BBB penetration, our GLS1 therapy acts in the bloodstream, depleting glutamine systemically, thereby impairing tumor growth and survival.
Mechanism of Action
GBM’s Glutamine Addiction:
- Tumor cells lack glutamine synthetase and overexpress glutamine transporters, making them entirely dependent on external glutamine uptake.
- Systemic Glutamine Depletion: GLS1 enzymatically degrades circulating glutamine, disrupting tumor metabolism and causing cell death.
- Selective Tumor Starvation: Healthy cells, which retain the ability to synthesize glutamine, remain unaffected, significantly reducing systemic toxicity.
Preclinical Validation
✔ In Vitro Efficacy
- GLS1 therapy significantly induced GBM cell death and suppressed proliferation in multiple GBM cell lines.
- Metabolic analysis confirmed disruption of glutamine-dependent pathways essential for tumor survival.
✔ In Vivo Efficacy (Murine GBM Model)
- GLS1-treated mice exhibited extended survival, with median survival increasing from 27 to 56 days (p = 0.0212).
- Tumor volume was reduced, and tumor vascularization decreased, suggesting impaired tumor angiogenesis.
- Histopathology confirmed a significant reduction in tumor cell mitotic activity.
✔ Safety Profile
- No morphological alterations were observed in liver and kidney tissues, indicating minimal systemic toxicity.
- Treated mice exhibited lower weight loss, suggesting reduced cachexia and improved overall physiological health.
Figure 1. Taking advantage of glioblastoma (GBM) dependence on glutamine to designe a metabolism-based therapy. The depletion of GBM cell line culture media and the systemic conditioning of murine GBM models induce a metabolic remodeling that prompts decreased GBM cell survival and tumor volume with a concomitant increased survival of the animal models.
STAGE OF DEVELOPMENT
The technology is at TRL 3 – Initial proof of concept demonstrated with a limited number of in vitro and in vivo models.
- In vitro efficacy confirmed in multiple GBM cell lines.
- In vivo efficacy validated in an orthotopic murine GBM model, with prolonged survival and reduced tumor burden.
- Favourable safety profile observed, with no significant off-target toxicity.
Next steps include preclinical validation (TRL 4–5), focusing on dose optimization, extended safety profiling, and regulatory alignment for clinical translation.
APPLICATIONS
- Glioblastoma Treatment – First-line or combinatory therapy in GBM management.
- Other Glutamine-Dependent Cancers – Potential for lung, pancreatic, and colorectal cancers, where glutamine transporter overexpression and glutamine synthase silencing indicate metabolic reliance.
- Neurodegenerative Disorders – Investigating applications where alterations in glutamine metabolism contribute to disease progression.
KEY ADVANTAGES AND BENEFITS
- No BBB Penetration Required – Effectively starves tumors without the need to cross the blood-brain barrier.
- Selective Tumor Targeting – Exploits a metabolic vulnerability unique to GBM cells.
- Minimal Systemic Toxicity – GLS1 therapy is well-tolerated, sparing healthy cells while disrupting tumor metabolism.
- Broad Oncology Potential – Can be applied to other glutamine-dependent cancers, such as lung, pancreatic, and colorectal cancer.
Current GBM therapies face limited efficacy, significant side effects, and high relapse rates due to BBB restrictions and tumor metabolic adaptability. Our systemic GLS1 therapy provides multiple advantages over existing solutions:
- Enhanced Survival Potential – Directly disrupts GBM’s metabolic reliance on glutamine, potentially prolonging patient survival.
- Targeted Metabolic Intervention – Unlike intracellular glutaminase inhibitors or glutamine transport blockers, our therapy bypasses the BBB and systemically eliminates glutamine availability, depriving tumors of a key nutrient.
- Minimized Toxicity – Healthy cells are spared, avoiding severe side effects observed in previous systemic approaches like glutaminase-asparaginase hybrids, which failed due to toxicity.
- Novel systemic metabolic approach – Unlike existing metabolic therapies, which attempt to inhibit intracellular pathways, GLS1 therapy eliminates glutamine at its source, making it a first-in-class metabolic disruption approach for GBM.
INTELLECTUAL PROPERTY
- WO2025057133 (Priority Date: 14/09/2023)
FURTHER DETAILS
Martins, F., van der Kellen, D., Gonçalves, L. G., & Serpa, J. (2023). Metabolic Profiles Point Out Metabolic Pathways Pivotal in Two Glioblastoma (GBM) Cell Lines, U251 and U-87MG. Biomedicines, 11(7), 2041. https://doi.org/10.3390/biomedicines11072041
Nunes, S. C., Sousa, J., Silva, F., Silveira, M., Guimarães, A., Serpa, J., Félix, A., & Gonçalves, L. G. (2023). Peripheral Blood Serum NMR Metabolomics Is a Powerful Tool to Discriminate Benign and Malignant Ovarian Tumors. Metabolites, 13(9), 989. https://doi.org/10.3390/metabo13090989
Brito, C., Azevedo, A., Esteves, S. et al. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer 19, 968 (2019). https://doi.org/10.1186/s12885-019-6177-0
Pinho JO, Matias M, Marques V, Eleutério C, Fernandes C, Gano L, Amaral JD, Mendes E, Perry MJ, Moreira JN, Storm G, Francisco AP, Rodrigues CMP, Gaspar MM. Preclinical validation of a new hybrid molecule loaded in liposomes for melanoma management. Biomed Pharmacother. 2023 Jan;157:114021. doi: 10.1016/j.biopha.2022.114021. Epub 2022 Nov 16. PMID: 36399831.
OPPORTUNITY
- We are actively seeking strategic partnerships for:
- Co-Development Partnerships – Collaborating with pharmaceutical and biotechnology companies specializing in biologic therapeutics to advance the preclinical and clinical development of GLS1 therapy.
- Licensing Agreements – Technology available for licensing for clinical translation and commercialization.
NOVA Inventors
Jacinta Serpa
Filipa Martins
David Van der Kellen
Fernanda Silva
Sofia Fernandes