
Tailored People-centric Solutions to Foster Drug Development, Research, Science Communication and Health Literacy
CDG&Allies provides tailored people-centric solutions to foster drug development, research, science communication and health literacy
Background
Engaging patients (and non-experts in general) in scientific research is becoming common practice and a regulatory demand. This is particularly important in rare diseases given the specificity of these disorders, the scarcity of patients, samples and reliable information. However, patient engagement implementation often lacks effective and proven strategies, especially during the early phases of drug development.
Technology Overview
CDG & Allies has developed an efficient and adaptable patient-centric framework to accelerate research, support biotech & pharmaceutical companies, healthcare systems and patient associations interested in driving research. Its main focus are rare diseases, with Congenital Disorders of Glycosylation (CDG) being used as a model. A varied range of competitively priced and custom-made services targeting and involving various audiences – i) research, ii) patient and iii) general community – have been created. Our solid scientific know-how, international experience have allowed us to create an innovative portfolio which continues to expand. Additionally, the versatile and adjustable nature of our methodology makes it transposable to other rare, chronic or more common diseases, such as cancer.
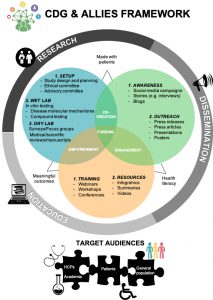
Fig. 1: CDG & Allies Framework.
Further Details:
- D Marques-da-Silva, V Dos Reis Ferreira, M Monticelli et al., Liver Involvement in Congenital Disorders of Glycosylation (CDG). A Systematic Review of the Literature, J Inherit Metab Dis, 2017 Mar;40(2):195-207. doi: 10.1007/s10545-016-0012-4
- C de Freitas, V dos Reis Ferreira, S Silva et al., Public and patient involvement in needs assessment and social innovation: a people-centred approach to care and research for congenital disorders of glycosylation. BMC Health Serv Res. 2017; 17: 682. doi: 10.1186/s12913-017-2625-1
- R Altassan, R Péanne, J Jaeken et al., International Clinical Guidelines for the Management of Phosphomannomutase 2-congenital Disorders of Glycosylation: Diagnosis, Treatment and Follow Up. J Inherit Metab Dis, 2019 Jan;42(1):5-28. doi: 10.1002/jimd.12024.
- Rita Francisco, Carlota Pascoal, Dorinda Marques-da-Silva, Sandra Brasil, Fernando M. Pimentel-Santos, Ruqaiah Altassan, Jaak Jaeken, Ana Rita Grosso, Vanessa dos Reis Ferreira, Paula A. Videira. New insights into immunological involvement in Congenital Disorders of Glycosylation (CDG) from a people-centric approach Accepted Manuscript ID: jcm-833871 Journal of Clinical Medicine.
Stage of Development
- TLR 4/5 – In vitro drug/compound testing using patient-derived cells; Patient engagement in research, health literacy and science communication.
Benefits
- a broad range of services that ensure an early and continued patient involvement and sustained patient engagement in research. Additionally, good knowledge of patient needs is in accordance with regulatory agencies (EMA, FDA) guidance which can facilitate and accelerate the regulatory evaluation process;
- an international scope;
- a highly versatile and result-driven methodology.
Applications
- Patient engagement in early-stage research and throughout different phases of clinical development (from early stages to regulatory evaluation);
- In vitro drug efficacy testing for the development of novel therapies or proof-of-principle studies;
- Awareness, dissemination and educational campaigns in the medical/research field targeting the patient, general and research populations.
-
Health literacy promotion and consultancy (educational and awareness content development and dissemination (e.g.social media campaigns, press release, webinars);
-
Collecting of patient-fed data through surveys (expertise in survey development, dissemination and administration and analysis);
-
Patient-centric research design (patient recruitment, advisory committees and focus groups set-up, ethical committee facilitation, result dissemination);
-
Scientific events (e.g. conferences and workshops) organization;
-
In vitro testing in appropriate disease models (experience with cellular models, drug/compound testing, comparative transcriptome analysis).
- Medical articles, letters, reports or abstracts and posters;
- Medical guidelines preparation;
- Preparation, revision and submission of funding applications;
- Ethical committee document preparation and submission;
- Lay language materials (summaries, infographics, videos);
- Interviews, press releases, press articles;
- Social media posts;
- Website population and content-building.
- Viability testing;
- Effective dose;
- Target validity;
- Comparative Transcriptomic Analysis;
- Functional assay.
- R software for appropriate bioinformatics and statistical methods;
- Survey Monkey/Google Forms;
- NVivo;
- Weebly;
- Canva.
Opportunity
- World CDG Organization Platform: Worldcdg.org;
- Social media channels and campaigns (World CDG Awareness Day campaign (see video); )
- World CDG Conference for Families and Professionals (4 editions have been organised; 5th edition is under preparation);
- Lay language materials (For examples see Rare CDG infographics series, Glycoland fairy tale).
Intellectual Property
- Know-how based.
Research Unit
UCIBIO.
NOVA Inventors
Paula Alexandra Videira


