
Green syngas from carbon dioxide
A concept of electrodes based on non-noble metals that can produce syngas with a tuneable composition.
BACKGROUND
Carbon capture and utilization (CCU) technologies are being intensively investigated, such as power to gas and power to liquid, to produce fuels and chemical building blocks from CO₂. In a recent report it was shown that CCU has the technical potential to change the paradigm for chemical production based on fossil resources to renewable energy, reducing annual greenhouse gas (GHG) emissions by up to 3,5 Gt CO₂-eq in 2030.
In all scenarios proposed to mitigate CO₂ emissions, the capture and storage of carbon dioxide plays an important role. As a consequence of the predicted large scale application of these techniques, high pressure CO₂ will become available for all potential users in much higher quantities and lower prices than current ones. All kinds of carbon dioxide uses may potentially benefit from this situation.
Electrochemical CO₂ reduction is a CCU technology. In this process, carbon dioxide is co-electrolyzed with water near room temperature. Hydrogen is produced in situ. Depending on process conditions and catalysts, CO₂ reduction originates a series of products, such as CO, formic acid, hydrocarbons, alcohols, etc. at the cathode. Oxygen is produced at the anode resulting from water oxidation reaction.
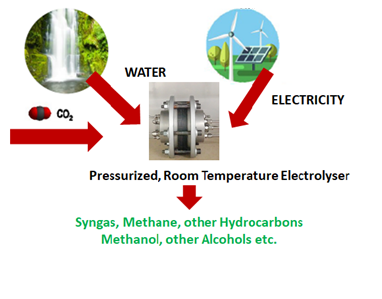
We are developing a unique technology for producing pressurized green syngas (CO+H₂) electrochemically. Green syngas produced using renewable energy can be an important platform for introducing renewable energy in the chemical industry.
TECHNOLOGY OVERVIEW
- Researchers at NOVA have developed a concept of electrodes based on non-noble metals that can produce syngas with a tuneable composition (e.g. H₂:CO 2:1 to produce methanol). The use of non-noble metals contributes to the cost effectiveness of this solution (Figure 1);
- The proprietary catalytic system based on a catalytic cathode and an ionic liquid containing electrolyte enhances CO₂ absorption and consequently the productivity of the process. It increases simultaneously its energy efficiency (decreasing the overpotential of the electrochemical reaction);
- The use of pressures higher than atmospheric pressure allows a further increase in productivity and energy efficiency by minimizing intermediary steps of compression of gases for downstream processing and storage;
- Potential total CO₂ conversions can be achieved, minimizing the costs of separation of unreacted CO₂;
- This flexible CO₂ electrolyser can be applied at the place, where CO₂ is emitted, or at a place where CO₂ is stored;
FURTHER DETAILS
The work related to the proof of concept of the technology and scale-up investigations in a laboratory prototype, designed and built on purpose for this application was published in the following papers:
- Pardal, T.; Messias, S.; Sousa, M.; Reis-Machado, A.S.; Rangel, C.M.; Nunes, D.; Pinto, J.V.; Martins, R.; Nunes da Ponte, M. Syngas production by electrochemical CO₂ reduction in an ionic liquid-based electrolyte. J CO₂ Utilization 2017, 18:62–72. Doi: 10.1039/c9re00271e;
- Messias, S.; Sousa, M.M.; Nunes da Ponte, M.; Rangel, C.M.; Pardal, T.; Reis-Machado, A.S. Electrochemical production of syngas from CO₂ at pressures up to 30 bar in electrolytes containing ionic liquid. React. Chem. Eng. 2019, 4, 1982–1990. Doi: 10.1039/C9RE00271E;
STAGE OF DEVELOPMENT
TRL 3-4 – Research and Development is ongoing.
BENEFITS
Currently there are no industrial CO₂ electrolysers. This technology could provide synthetic sustainable fuels and chemical building blocks to feed a circular economy.
APPLICATIONS
End-users of the technology:
- CO₂ emitters industry;
- Electrolyser market;
- Fuel market for transportation, residential and industrial deployment;
- Renewable energy parks;
- Electric energy providers;
- Sustainable building blocks for the chemical industry.
OPPORTUNITY
The university would appreciate industrial partner guidance for further scale-up, and is also seeking funding for this purpose. Specifically, the university is interested in co-development partners for:
- further scale-up of the electrolyser;
- electrode development , in particular anodes based on non-noble metals;
- developers of ionic exchange membrane separators.
Intellectual Property
Background IP belongs to Omnidea, a spin-off from NOVA. There is much room for further IPR protection relative to new advances in the technology, which are being developed at NOVA, by NOVA Researchers.
NOVA Inventors
Ana Reis Machado
Sofia Alexandra Pereira Messias
Manuel Luis de Magalhães Nunes da Ponte
Zeljko Petrovski
Daniela da Silva Nunes Gomes
Rodrigo Ferrão de Paiva Martins


