Non-Invasive Urine Test for Prostate Cancer
Protein Signature for Accurate Detection of Prostate Cancer Using Urinary Extracellular Vesicles.
BACKGROUND
Prostate cancer (PCa) is the most diagnosed cancer and second leading cause of cancer-related death among men in developed countries. By 2030, 1.7 million new cases and 500,000 deaths are projected. Current screening tests like PSA have a high false-positive rate, leading to unnecessary procedures and treatments. Therefore, there is a critical need for more accurate, minimally invasive screening tests to improve early diagnosis, treatment, and patient quality of life.
TECHNOLOGY OVERVIEW
Researchers at NOVA Medical School have identified a panel of protein biomarkers derived from urinary extracellular vesicles (EVs) for the non-invasive assessment of clinically significant prostate cancer (PCa).
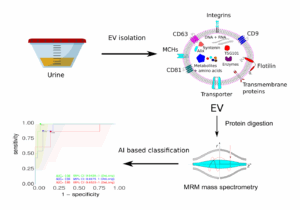
Through a structured discovery pipeline involving EV isolation, label-free liquid chromatography–mass spectrometry (LC-MS/MS), and differential expression analysis, a total of 171 proteins were found to be significantly associated with PCa.
The majority of these candidates are supported by prior literature, reinforcing the biological relevance of the panel. Importantly, several of the identified proteins are enriched in patients presenting with intraductal carcinoma (IDC) and cribriform (Crib) histologic patterns—features associated with aggressive disease and poor clinical outcomes.
A subset of high-confidence biomarkers is protected under a recent patent application, including: TMBIM1, GNG5, IGLL5, S100A10, PTGES3, C1QA, and FGG.
These proteins are implicated in key oncogenic pathways, including immune modulation, extracellular matrix remodeling, and vesicle-mediated signalling.
To support clinical translation, the team has developed a targeted quantification strategy using multiple reaction monitoring (MRM) and stable isotope-labelled peptides. This method enables sensitive and reproducible detection of selected biomarkers in urine samples and is compatible with high-throughput clinical assay development.
The complete biomarker panel, MRM transition data, and associated clinical annotations are available for collaborative research, assay development, and validation studies under confidentiality agreement.
Figure 1: Overview of the three steps included in the proposed kit.
STAGE OF DEVELOPMENT
TRL 2 — Proof of principle established.
APPLICATIONS AND BENEFITS
- Non-Invasive Sampling: Based on urine-derived extracellular vesicles (uEVs), enabling collection without the need for invasive procedures.
- Early Risk Stratification Potential: Biomarkers are enriched in patients with aggressive histologic patterns (IDC and Crib), which are often underdiagnosed in standard clinical workflows.
- Established Relevance: Over two-thirds of the identified proteins have been previously linked to prostate cancer, reinforcing the biological and translational potential of the panel.
- Assay-Ready Format: MRM transitions for selected peptides have been designed, supporting future development of high-precision quantification assays.
- Scalable Analytical Platform: MRM-based workflows using stable isotope-labelled peptides are compatible with automated sample preparation and high-throughput mass spectrometry.
- Foundation for Diagnostic Innovation: Offers a robust biomarker dataset to support the development of new liquid biopsy tools for prostate cancer detection and monitoring.
We foresee that the proposed solution may evolve into an in vitro diagnostic tool with stable isotope labelled reference peptides and a software that can analyse and classify patients’ prostate cancer prognosis based on urine samples and other readily available clinical parameters.
This IVD can be used for:
- Early Detection and Monitoring: For prostate cancer, particularly IDC and Crib patterns.
- Clinical Diagnostic Tool: For use in medical diagnostics to guide treatment decisions.
- Research and Development: For further exploration of prostate cancer biomarkers and therapeutic targets.
- Companion Diagnostics: To complement existing screening methods such as PSA testing.
INTELLECTUAL PROPERTY
- PCT/IB2025/051881 – PROSTATE CANCER BIOMARKERS, USES AND METHODS THEREOF
OPPORTUNITY
- The biomarker panel is available for licensing and collaborative development. We are actively seeking:
- Co-development partners for assay design (e.g. MRM or immunoassays) and biomarker validation in independent clinical cohorts
- Collaborators in software and data analytics for future integration of biomarker data with clinical parameters
- Translational research partners to support regulatory planning, sample processing, and multi-centre study design
- Industry stakeholders interested in incorporating this panel into broader liquid biopsy or prostate cancer diagnostic platforms
NOVA Inventors
Rune Matthiesen
Ana Sofia Carvalho