
PRISM™: Three-Dimensional Model of Early Diabetic Retinopathy
Human 3D retinal organoid mimicking early-stage diabetic retinopathy for 96-well therapeutic & gene-therapy screening, efficacy profiling and validation.
BACKGROUND
Unmet need — Early-stage diabetic retinopathy (DR) already threatens sight in 146 million people worldwide, yet no approved therapy targets this neuro-inflammatory phase when vision could still be saved.
Predictive gap — Rodent models do not develop DR naturally and 2D retinal cultures lack tissue layering, driving a 90 % clinical failure rate for ocular drugs.
Economic impact — Each unsuccessful DR candidate pushes the average cost of a successful ocular drug to US $ 879 million, slowing innovation and market entry.
TECHNOLOGY OVERVIEW
PRISM™ is a banked human hiPSC-derived 3-D retinal organoid that faithfully recreates the neuro-inflammatory phenotype of early diabetic retinopathy. In a 96-well format it delivers multi-parametric read-outs compatible with high-content imaging, qPCR, ELISA and Western-blot.
Key validated endpoints (vs. control)
| Endpoint | Assay | Fold-change vs control |
| Ganglion / amacrine survival | IF (BRN3a, AP2α) | -50 % |
| VEGF, IL-1β expression | qPCR | ↑ 2 × |
| MCP-1 secretion | ELISA | ↑ 1.75 × |
| ROS levels | DCF-DA | ↑ 1.6 × |
| mTOR activity | p-S6 WB | ↑ 2 × |
All endpoints reproduced across three independent differentiations (CV < 15 %).
A complete data package can be returned in under three weeks.
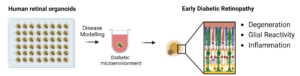
Fig. 1: Retinal organoids with a specific development stage are exposed to a diabetic microenvironment for an optimized period of time. These organoids develop features of early diabetic retinopathy such as neurodegeneration, glial reactivity and inflammation.
STAGE OF DEVELOPMENT
TRL: 3-4 (proof-of-concept complete).
BENEFITS & APPLICATIONS
Only validated model of early-stage DR – Rodents never develop pre-vascular DR, whereas PRISM reproduces the human neuro-inflammatory phase with -50 % ganglion/amacrine survival, 2 × VEGF / IL-1β, 1.75 × MCP-1 and 1.6 × ROS (patent data; n = 3 differentiations).
Weeks instead of months – high-glucose challenge delivers decision-making data in < 3 weeks, versus 12–16-week diabetic mouse protocols that model only late vascular damage.
Multi-endpoint in a single assay – High-content imaging, qPCR, ELISA and Western blot run on the same organoid plate, providing parallel efficacy and mechanism read-outs.
Animal-free & regulator aligned – Fully human 3-D system meets the direction of FDA Modernization Act 2.0 and EMA 3R roadmap, lowering ethical burden and translational risk.
Ready with commercial hiPSC lines – Uses off-the-shelf cell sources; no patient tissue or complex ethics approvals needed, simplifying tech transfer and future scale-up.
Phenotypic hit & lead screening – Rank neuro-protective or anti-inflammatory small-molecule libraries directly in human retinal tissue.
Mechanism-of-action validation – Quantify pathway modulation (mTOR, ROS, cytokines) to confirm target engagement before in-vivo studies.
Gene-therapy efficacy testing – Assess AAV or CRISPR constructs for rescue of ganglion/amacrine degeneration and inflammatory biomarkers.
Retina-specific safety profiling – Detect early neuro-toxicity or pro-inflammatory off-targets that 2-D cultures and rodent models miss.
Biomarker discovery support – Generate human retinal RNA/protein signatures to guide clinical translation and patient-stratification efforts.
FURTHER DETAILS
de Lemos, L., Antas, P., Ferreira, I. S., Santos, I. P., Felgueiras, B., Gomes, C. M., Brito, C., Seabra, M. C., & Tenreiro, S. (2024). Modelling neurodegeneration and inflammation in early diabetic retinopathy using 3D human retinal organoids. In Vitro Models, 3(1), 33–48. https://doi.org/10.1007/s44164-024-00068-1
INTELLECTUAL PROPERTY
- Tenreiro, S. I. N., de Lemos, M. L. M., & de Seabra, M. P. C. (2024). 3D cellular model of early diabetic retinopathy (EP23825089.8)
Priority date: 30 November 2022
OPPORTUNITY
- We are seeking:
- Non-GLP Pre-clinical contract studies – Engage PRISM as a fee-for-service platform to test partner compounds, biologics or AAV vectors.
Project timelines and pricing upon contact.
- Co-development partnerships – Joint projects to expand biomarker panels, microglia integration or automation for 96-well screening.
- Licensing outside DR – Exclusive or non-exclusive rights for other retinal indications (AMD, glaucoma) or for kit commercialisation.
NOVA Inventors
Sandra Tenreiro
Maria Luísa Machado
Miguel Seabra


