
Three-Dimensional Model of Early Diabetic Retinopathy
An in vitro cellular culture development for novel therapeutic testing/pre-clinical research for novel anti-eye diabetic drugs for DR
BACKGROUND
Diabetic retinopathy (DR) is a major complication of diabetes and a leading cause of visual impairment in the working-age population affecting ~30% of the patients after 5 years of diabetes diagnosis. The therapeutic strategies available for DR only target the later stages of the disease involving vascular defects such as macular edema or neovascularization. However, retinal neurodegeneration and inflammation is known to precede vascular alterations. Therefore, it is urgent to identify new therapeutic approaches acting on early stages and, consequently, halting disease progression.
The DR complex models developed so far involve animal experimentation and present several limitations on pre-clinical studies, increasing therefore the rate of failure during clinical stages, which ultimately translate into higher costs for a novel drug to treat and cure diabetic patients with visual loss.
TECHNOLOGY OVERVIEW
This technology is an in vitro cellular culture development for novel therapeutic testing/pre-clinical research for novel anti-eye diabetic drugs for diabetic retinopathy (DR) using differentiated retinal organoids from human induced Pluripotent Stem Cells (hiPSCs) to generate a disease model of early DR.
This 3D disease model reproduces the main molecular and cellular features of the early phase of DR. This disease-mimicking tool is extremely powerful for pharmaceutical drug development process targeting DR, from drug discovery or gene therapy to preclinical research being useful to select the most promising drug candidates for clinical trials.
In order to develop the in vitro disease model for DR, a previous established protocol for human retinal organoids was used as a starting point to differentiate hiPSCs and subsequently, generate a model of early DR by optimizing its exposition to a diabetic microenvironment.
Several cycles of optimization and validation were performed using conditions that induce the diabetic environment in cells and tissues. Hallmarks of early DR were used to fine-tune and to reach the best conditions that mimic the diabetic eye in the early stages of the disease, namely, the stage of retinal organoids development, the duration of the treatment and the concentration of glucose to be used were carefully optimized.
The product is formulated as a cryopreserved solution, for example, supplied in frozen cryovials; organoid lines ready for seeding into plate assays with growth conditions established with consistency and reproducibility.
With the model of the present invention, it is possible to reproduce established molecular and cellular features of early DR, such as the loss of ganglion and amacrine cells, the induction of glial reactivity and inflammation, together with the increase of VEGF and IL-1β expression and MCP-1 secretion. Moreover, increased levels of reactive oxygen species (ROS) accompanied by activation of key enzymes involved in antioxidative stress response is also obtained with the present model.
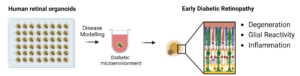
Fig. 1: Retinal organoids with a specific development stage are exposed to a diabetic microenvironment for an optimized period of time. These organoids develop features of early diabetic retinopathy such as neurodegeneration, glial reactivity and inflammation.
STAGE OF DEVELOPMENT
Technology Readiness Level (TRL): 3 – Experimental Proof of Concept
BENEFITS & APPLICATIONS
This 3D organoid-based technology is a better tool for companies targeting DR drug discovery or gene therapy and preclinical research as it presents the following advantages:
- the 3D in vitro model creates the cellular organization of the human retina in a diabetic eye, resulting in a remarkably accurate model for drug development that allows most promising candidates to be translated into clinical trials;
- human iPSCs from healthy individuals (commercially available) are used to induce the diabetic organoid, therefore existing no need for cells from diabetic patients with DR;
- the existence of an organoid-based technology for DR avoids and reduces the use of animals in pre-clinical studies. Moreover, the mouse eye is very different from the human eye, the present model providing a reliable model which mimics the human eye;
- the present technology is scalable: a large quantity of organoids that can be obtained from human iPSCs and created on a dish is unlimited when compared with commonly used animal models, limited to two eyes per animal;
- organoids disease models are an effective time- and cost‑ reducing tool in drug screening and preclinical research for the biotech and pharmaceutical industry.
The present disease-mimicking tool is extremely powerful for pharmaceutical drug development process targeting DR, from drug discovery or gene therapy to preclinical research.
Until this moment, there are 80+ active players working to develop DR treatment therapies, but do not exist commercially available in vitro 3D-organoid disease model for DR.
INTELLECTUAL PROPERTY
- EP22217331.2 (Priority date: 30/11/2022).
- PCT/IB2023/062075
OPPORTUNITY
- Development partner which could help to reach the technology as a final product (e.g. Organoid companies);
- Licensing (exclusive and non-exclusive) after having the technology as a final product. For this, the inventors need to perform some tests and validations.
NOVA Inventors
Sandra Tenreiro
Maria Luísa Machado
Miguel Seabra


